Radiation Dosimetry

In 1895, Welhelm Roentgen discovered the x-ray. Ever since discovering a ray that could pass through solid material, there has been a method to view the human body. However, from this discovery, there was also a need to monitor and count the amount of radiation an individual was exposed to. Over the next half century, physicists developed many methods for determining how much radiation one is exposed to. The emerging nuclear physics field brought many new terms to explain what was happening to create radiation, how it interacted with matter, and the processes in which we measured and recorded the interactions.
Madam Curie, for which the units of activity are named, became renowned for her discovery of Polonium and Radium in the early 1900s. However, her discovery of radioactivity came from the initial findings of Antoine Henri Becquerel. He placed a chunk of uranium-bearing crystal onto a photographic plate after having exposed the crystal to direct sunlight. After some time, the ore has produced an image on the plate. The release of X-rays from the crystal and their subsequent recording on the plate was the first method of recording radiation. Although crude, this method allowed doctors, shoe sales clerks, and anyone interested in photographing the skeletons of human beings the opportunity to do so. Despite the thrilling results achieved from a physical picture relating to exposure, a more systematic and practical approach was taken.

The electrical approach was preferred over the photographic plate due to the immediate results. Initially, the amount of radiation in the environment was calculated by measuring the conductivity of the air. However, there was disagreement about what the standard should be.
Eventually, the scientific community agreed upon the Curie, defined as the quantity of radon in equilibrium with 1g of radium. This standard was then used to propose the electrostatic unit, defined as that quantity of x-radiation that liberates by ionization one electrostatic unit of electricity per cubic centimeter of air under normal temperature and pressure conditions. Later, in 1914, William Duane defined the dose as the intensity of radiation multiplied by the time in seconds to the exposure to the radiation beam. These standards set the stage for various devices that were to prove instrumental in keeping members of the medical profession safe, and later those in the nuclear industry.
Although early forms of dosimetry involved measuring the conductivity of the air, it was quickly discovered that to measure individual doses, the measuring device must be able to be worn on the worker in question. This led to personal dosimetry that used gels. The gels would change colors as they were exposed to radiation. After the gel underwent a complete transformation, the individual received a known exposure.
Another method of measurement was that of calorimetry. As the exposure increased, so did the temperature of the monitoring device. These devices could not provide a reliable readout since they were susceptible to environmental and user errors. The need for a dosimetry device that was accurate, precise, and able to be worn necessitated the changes to modern dosimetry.
Modern dosimetry takes several forms. As technology has developed, the monitoring schemes used for radiation workers have also changed over time.
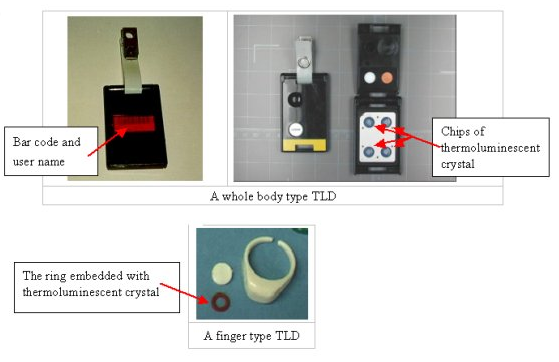
During the latter part of the last century, doped crystal thermoluminescent dosimeters and electrostatic reed self-indicating dosimeters were particularly popular. Doped crystal dosimeters work by doping a crystal matrix with impurities. When incident gamma rays interact with the calcium (or other element) crystal, electron pairs are created. These electron pairs are electrostatically stored within the impurities. The crystal is contained within a nitrogen-filled glass bulb and wrapped in a gold wire.
This setup allows a current to be run through the wire, releasing the electrostatic charge stored within the impurities. By doing this procedure in a darkened photomultiplier tube, a slight luminescence of the crystal can be observed and recorded. The intensity and duration of luminescence are used to create a chart that can be directly correlated to the amount of gamma-ray exposure the crystal has undergone. Extrapolation yields the radiation exposure the wearer of the thermos-luminescent dosimeter has undergone.
Another type of dosimeter often worn by radiation workers is an accident dosimeter. These are used to record radiation exposure due to a nuclear accident. Due to their relatively small size, many thermo-luminescent dosimeters carry an accident dosimeter onboard. Accident dosimeters are often a simple arrangement of boron pellets and indium foil. Normally, neutron exposure is not expected for radiation workers. However, during a nuclear accident, high neutron flux levels may be expected following a criticality accident.
In an accident dosimeter, the indium foil undergoes transmutation when struck by a fast neutron. The foil's transmutation can be correlated to an approximate level of fast neutron exposure. Similarly, boron pellets register the amount of thermal neutron exposure personnel have undergone. Boron is chosen because it can absorb six thermal neutrons before becoming unstable. By measuring the accident dosimeters around a criticality accident, forensic analysis can help reconstruct details of the explosion when other methods would fail.
When a radiation worker plans on entering a high radiation area, the worker will be expected to wear some form of self-indicating dosimetry. Low-tech self-indicating dosimeters are still commonly used as a backup method for ensuring local radiation exposure limits are not exceeded.

These simple dosimeters are composed of a quartz reed placed against a scale and coupled to an electrode set that runs throughout the length of the dosimeter. A small electrostatic charge is applied to align the reed with the chart's zero exposure spot. As radiation is received, the ionization the electrodes receive will lower the electrostatic charge level of the reed. This causes the reed to deflect less and less. As a result, the indicated exposure level will rise. Quartz reed dosimeters are prone to errors from parallax error, moisture contamination, bumping, and charge decay. Because of this, they are gradually being phased out for electronic personal dosimeters.
Another popular personal dosimeter in everyday use is the film badge dosimeter. These are gradually being phased out for more accurate detectors but still see common use due to extremely low cost. In a film badge dosimeter, photographic film is placed on a badge holder. As the photographic film absorbs ionizing radiation, it gradually darkens by measuring the opacity of the film, and an approximate radiation exposure level can be recorded. The primary use of these dosimeters has slowly shifted to individuals not expected to receive any radiation exposure as part of their occupation. Any unexpected darkening of the film indicates an unexpected radiation leak.
Modern dosimeters typically incorporate solid-state technology to achieve a much more accurate reading. Electronic personal dosimeters use metal oxide semiconductor field effect transistors (MOSFETs). Incident gamma rays cause ionization to occur in the semiconductor substrate. This causes the electron carrier to deficit charge carrier ratio to change, which alters the conduction voltage necessary for the MOSFET to allow current flow. This conduction voltage change is proportional to radiation exposure. Incorporating this into an alarm circuit allows the user to set an audible alarm as radiation limits are approached. Furthermore, adding a seven-segment display with analog-to-digital converter circuitry allows the user to observe radiation exposure levels continuously.

As technology and understanding of the modern world advance, the need to monitor exposure to possibly deadly radiation levels arose. Early pioneers in the field unknowingly put their lives in harm's way to advance the study and understanding of nuclear physics. Today, methods and controls, including personal dosimetry, are in place to ensure the safe handling, testing, and experimentation of radioactive materials. Today's dosimetry devices provide fast, accurate, and reliable methods to calculate and record a person's exposure to radiation and allow employers and governments to protect their workers to the best of their abilities.





Member discussion